1. Decontamination mechanism using low surface tension
Use cleaning agents with low surface tension to act on the surface of the object to be cleaned and the interface it contacts to remove dirt, as shown in Figure 1. When the cleaning agent with a smaller surface tension than the A and B layers contacts the interface of the cleaned A and A and B layers, it will penetrate and diffuse on the A and B interfaces to peel off the A and B layers. Since the surface tension of dirt is generally much greater than 30N/m, if a cleaning agent with a lower surface tension is used, it has the cleaning function of directly peeling off the dirt B from the object to be cleaned A.
Dirt cleaning mechanism of low surface tension cleaning agents
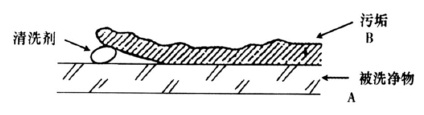
Figure 1 shows the dirt cleaning mechanism of low surface tension cleaning agents
2. Decontamination mechanism using surface active energy emulsification, dispersion and solubilization
Water-based cleaning agents mainly use the wetting, penetration, emulsification, dispersion and solubilization properties of SAA. Although there is adhesion between the dirt and the workpiece, and there is surface tension between the oil and water, the wetting and penetration of the cleaning agent will reduce the adhesion, the emulsification of the cleaning agent will reduce the surface tension at the junction of the oil and water, and the dispersing effect of the cleaning agent will disperse the carbon powder, dust, and metal powder in the dirt into many particles. The emulsification and solubilization of the cleaning agent can effectively prevent the dirt molecules from re-condensing and no longer depositing on the surface of the workpiece. If heat, mechanical stirring and jet are used, the dirt can be separated from the surface of the workpiece and rolled into the clear liquid to achieve the purpose of removing the dirt.
The mechanism of surfactant removing oily dirt is shown in Figure 2.
Cleaning mechanism of surfactant cleaning agent
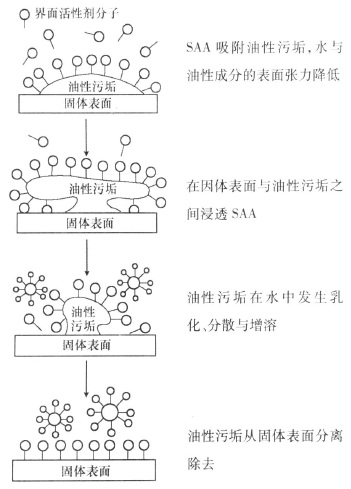
Figure 2 shows the cleaning mechanism of surfactant cleaning agent
3. Decontamination mechanism using chemical reaction dissolution separation
(1) Inorganic acid
Inorganic acids such as hydrochloric acid and nitric acid ionize a large amount of H+, and the following chemical dissolution reaction may occur during the cleaning process:
Cleaning mechanism of inorganic acid
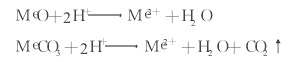
Figure 3 shows the cleaning mechanism of inorganic acid
(2) Organic acid
Representative ones are citric acid and ethylenediaminetetraacetic acid (EDTA). By using their own acidity and the excellent chelating ability of the active groups they carry, they can chelate, dissolve and disperse special dirt such as the oxide layer attached to the metal surface into the cleaning liquid to achieve the cleaning purpose.
(3) Alkali
Representative ones are sodium hydroxide, sodium carbonate, sodium phosphate and sodium silicate. Various alkalis can dissociate and remove dirt through saponification, conversion, neutralization and chelation separately or in combination.
(4) Oxidation-reduction agent
Use halogen and its compounds such as sodium hypochlorite and peroxide such as hydrogen peroxide to dissolve dirt through oxidation-reduction reaction, transform and remove it.
4. Mechanism of decontamination by electrolysis
Electrolytic cleaning agents are mainly composed of alkaline inorganic substances and can be divided into non-chelating type and chelating type. From the method of use, they can be divided into anodic electrolysis, cathodic electrolysis and PR electrolysis (anode and cathode electrolysis exchange treatment). The mechanism is mainly to remove oily dirt on the surface of the workpiece by oxidation (anodic electrolysis) and hydrogen (cathode electrolysis).


